In December 2015, Facebook founder Mark Zuckerberg and his wife Priscilla Chan announced their plan to put 99% percent of their Facebook shares, worth about 45 billion dollars, into a new project focusing on the improvement of human potential and the promotion of equality. After an initial focus on education, the initiative has now announced its second focal point: the promotion of basic science research with the goal of curing, preventing or managing all disease by the end of this century. This new project, led by neuroscientist Cori Bargmann, was announced in San Francisco yesterday. In addition to the President, the two founders also introduced the scientists who will help build this initiative in the future. The panel of experts also includes one German researcher: Tobias Bonhoeffer, Director at the Max Planck Institute of Neubiology.
Shortly after the birth of their daughter Maxima Chan Zuckerberg, the couple set up the Chan Zuckerberg Initiative, stating they wished to see their daughter grow up in a world in which it was possible to cure diseases, personalize learning, and connect people. At a press conference on the Mission Bay campus of the University of California in San Francisco yesterday, Chan and Zuckerberg announced the second cornerstone of the initiative: 'Chan Zuckerberg Science'.
Over the past few months, the paediatrician Chan and Facebook founder Zuckerberg had met with numerous scientists, engineers, and other experts. This convinced them of the importance to invest into basic research to achieve much needed scientific progress essential for combating diseases. As a result, they decided to set up "Chan Zuckerberg Science (CZS)". The work of the initiative will have three core elements: More and better cooperation of scientists and engineers, the development of new methods and technologies, and educating the general public about the benefits of scientific discovery with the result of a much more general support and willingness to donate to science.
The only European member of the Scientific Advisory Board is Max Planck Director Tobias Bonhoeffer from Martinsried. "I am truly thrilled to be part of this exciting initiative. I am convinced that CZS can provide a powerful new impetus for imaginative and forward-looking science in the biomedical arena", was his first reaction. "It is a great pleasure and honor for me to have been appointed as advisor," said Bonhoeffer, who had been in contact with Chan and Zuckerberg since their visit to Berlin earlier this year. Being involved in major science policy decisions is however nothing new for the 56-year-old neurobiologist Tobias Bonhoeffer. In his capacity as chairman of the Biology and Medicine Section of the Max Planck Society (2008 - 2011) and as a Governor of the Wellcome Trust, one of the world's largest charities, he has provided guidance and expertise to many decision-making bodies.
Read more


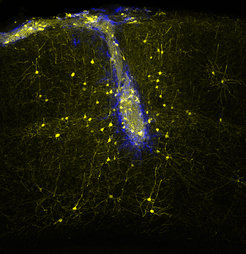

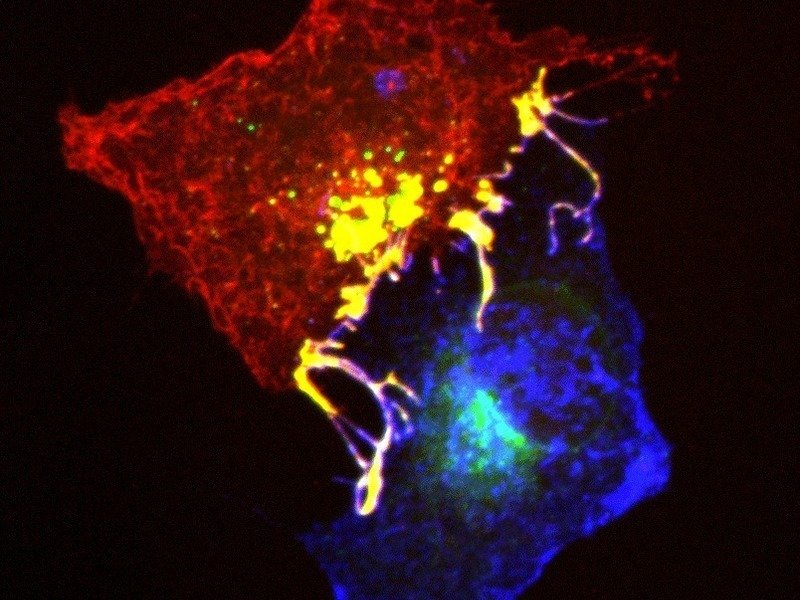
 Many diseases also have a genetic component. For example, gene mutations can lead to the development of cancer or psychiatric disorders such as schizophrenia, depression or autism. Most of these diseases seem to be caused not by a single mutation but are rather the result of the interplay of dozens, maybe even hundreds of altered gene functions. It would therefore be of great benefit for the investigation, diagnosis and therapy of these illnesses, to identify the affected genes and monitor their activity in the brain of the living organism.
Many diseases also have a genetic component. For example, gene mutations can lead to the development of cancer or psychiatric disorders such as schizophrenia, depression or autism. Most of these diseases seem to be caused not by a single mutation but are rather the result of the interplay of dozens, maybe even hundreds of altered gene functions. It would therefore be of great benefit for the investigation, diagnosis and therapy of these illnesses, to identify the affected genes and monitor their activity in the brain of the living organism.