Vincenz-Donnelly, L., Holthusen, H., Korner, R., Hansen, E.C., Presto, J., Johansson, J., Sawarkar, R., Hartl, F.U., and Hipp, M.S.
EMBO J, 2017, [Epub ahead of print].
doi 10.15252/embj.201695841
High capacity of the endoplasmic reticulum to prevent secretion and aggregation of amyloidogenic proteins
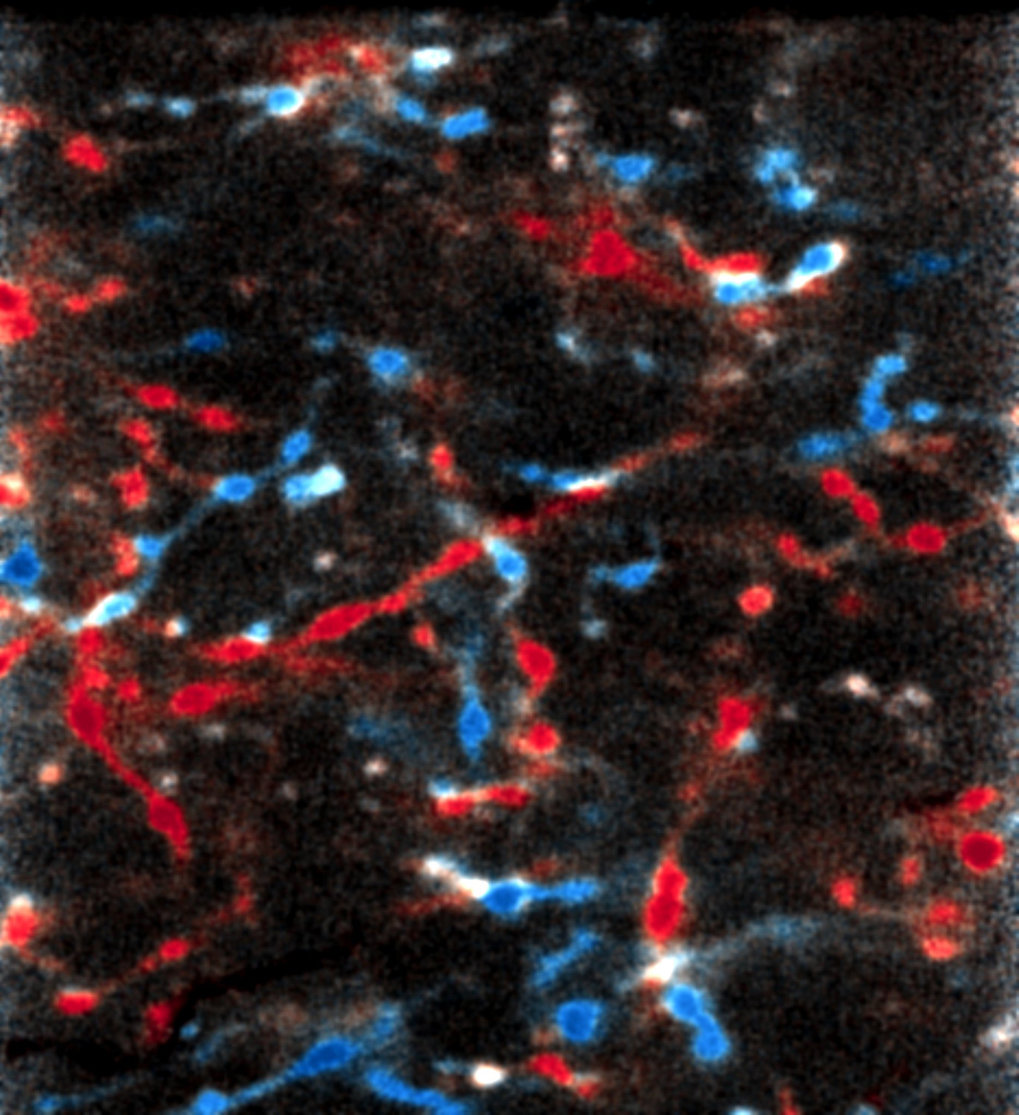
Protein aggregation is associated with neurodegeneration and various other pathologies. How specific cellular environments modulate the aggregation of disease proteins is not well understood. Here, we investigated how the endoplasmic reticulum (ER) quality control system handles β-sheet proteins that were designed de novo to form amyloid-like fibrils. While these proteins undergo toxic aggregation in the cytosol, we find that targeting them to the ER (ER-β) strongly reduces their toxicity. ER-β is retained within the ER in a soluble, polymeric state, despite reaching very high concentrations exceeding those of ER-resident molecular chaperones. ER-β is not removed by ER-associated degradation (ERAD) but interferes with ERAD of other proteins. These findings demonstrate a remarkable capacity of the ER to prevent the formation of insoluble β-aggregates and the secretion of potentially toxic protein species. Our results also suggest a generic mechanism by which proteins with exposed β-sheet structure in the ER interfere with proteostasis.